Metal-Ligand Multiple Bonds
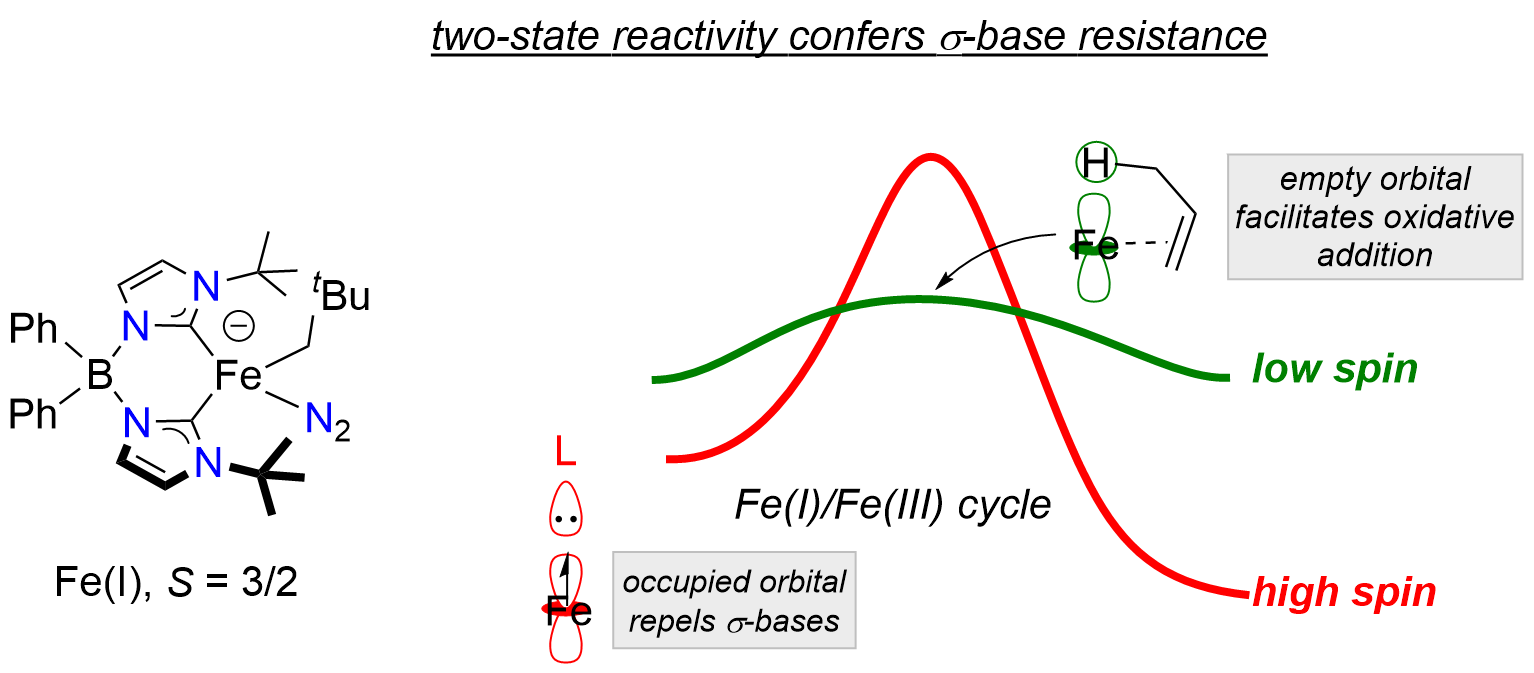
Metal-ligand multiple bonds are associated with diverse reactivity patterns, including transformations that are applicable to atom-economical catalysis, e.g. atom/group transfer reactions. The redox-richness of base metal complexes (e.g. Mn, Fe, Co), coupled with their ability to access multiple spin states, is expected to afford metal-ligand multiple bond complexes with unusual electronic structures and reactivity patterns.
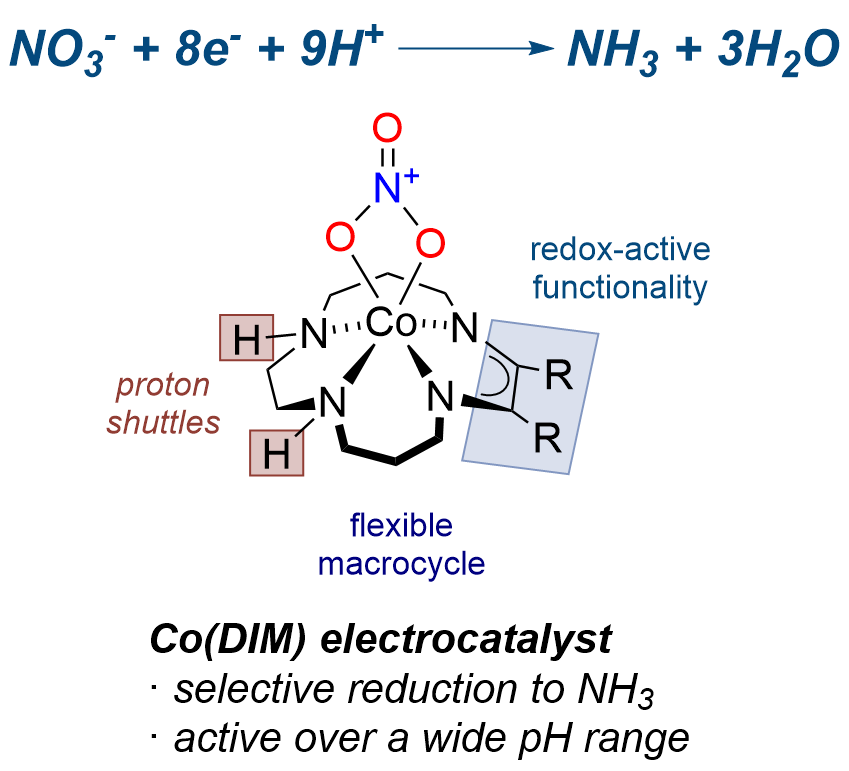
We target metal-ligand multiple bonds in base metal complexes that are supported by strongly-donating, multidentate carbene ligands. These modular ligands provide a high degree steric and electronic flexibility, and importantly create an ideal framework for stabilizing reactive metal-ligand multiple bonds, including for atomic ligands.
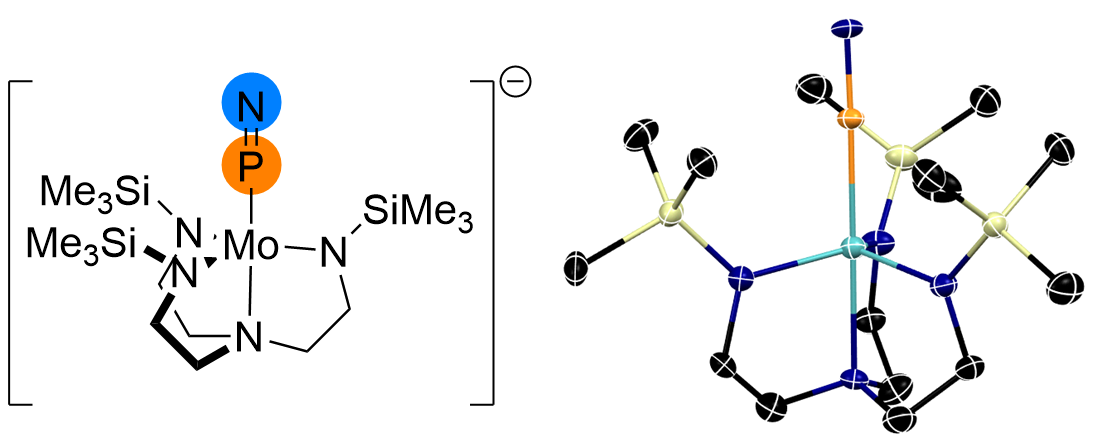
Our group has pioneered the chemistry of iron(IV) nitrido complexes supported by bulky tris(carbene)borate ligands, and have recently extended these to other single atom ligands. These complexes are reactive in multielectron reactions, including nitrogen atom transfer to a range of hydrocarbon substrates. We have also used these complexes as synthons in the assembly of new ligands, including the first structurally-characterized example of the interstellar gas, P≡N.